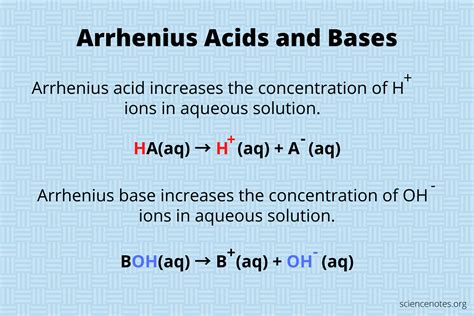
The concept of acids and bases is fundamental to chemistry, and one of the earliest and most influential definitions of an acid was provided by Svante Arrhenius, a Swedish chemist and Nobel laureate. In 1887, Arrhenius proposed that an acid is a substance that increases the concentration of hydrogen ions (H+) in a solution. This definition, known as the Arrhenius definition, marked a significant milestone in the understanding of acid-base chemistry. According to Arrhenius, acids are substances that dissociate in water to produce hydrogen ions, while bases are substances that dissociate in water to produce hydroxide ions (OH-).
The Arrhenius definition was groundbreaking because it provided a clear and concise way to distinguish between acids and bases. It also laid the foundation for later theories, such as the Bronsted-Lowry and Lewis definitions, which expanded our understanding of acid-base chemistry. However, the Arrhenius definition has some limitations, as it only applies to aqueous solutions and does not account for the behavior of acids and bases in non-aqueous solvents. Despite these limitations, the Arrhenius definition remains an important concept in chemistry, particularly in the context of aqueous solutions.
Key Points
- The Arrhenius definition states that an acid is a substance that increases the concentration of hydrogen ions (H+) in a solution.
- Acids dissociate in water to produce hydrogen ions, while bases dissociate in water to produce hydroxide ions (OH-).
- The Arrhenius definition only applies to aqueous solutions and does not account for the behavior of acids and bases in non-aqueous solvents.
- The Arrhenius definition is an important concept in chemistry, particularly in the context of aqueous solutions.
- The definition laid the foundation for later theories, such as the Bronsted-Lowry and Lewis definitions, which expanded our understanding of acid-base chemistry.
Arrhenius Acid Properties

Arrhenius acids have several characteristic properties that distinguish them from bases. One of the most important properties of an Arrhenius acid is its ability to donate a proton (H+), which is a fundamental aspect of acid-base chemistry. Arrhenius acids also tend to be corrosive and can react with metals to produce hydrogen gas. Additionally, Arrhenius acids typically have a sour taste and can change the color of certain indicators, such as litmus paper.
Some common examples of Arrhenius acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3). These acids are all strong acids, meaning that they completely dissociate in water to produce hydrogen ions. Weak acids, on the other hand, only partially dissociate in water and include substances such as acetic acid (CH3COOH) and carbonic acid (H2CO3).
Arrhenius Acid Dissociation
The dissociation of an Arrhenius acid in water is a critical aspect of acid-base chemistry. When an Arrhenius acid is added to water, it dissociates into its constituent ions, releasing hydrogen ions (H+) into the solution. This process can be represented by the following equation: HA → H+ + A-, where HA is the acid and A- is the conjugate base. The extent of dissociation depends on the strength of the acid, with strong acids dissociating completely and weak acids dissociating only partially.
| Acid | Dissociation Constant (Ka) |
|---|---|
| Hydrochloric acid (HCl) | 1.3 x 10^6 |
| Sulfuric acid (H2SO4) | 1.0 x 10^2 |
| Nitric acid (HNO3) | 2.4 x 10^1 |
| Acetic acid (CH3COOH) | 1.8 x 10^-5 |

Arrhenius Base Definition

In addition to defining acids, Arrhenius also defined bases as substances that increase the concentration of hydroxide ions (OH-) in a solution. According to Arrhenius, bases are substances that dissociate in water to produce hydroxide ions, which can then react with hydrogen ions to form water. The Arrhenius definition of a base is closely related to the definition of an acid, as the two are intimately connected in acid-base chemistry.
Some common examples of Arrhenius bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2). These bases are all strong bases, meaning that they completely dissociate in water to produce hydroxide ions. Weak bases, on the other hand, only partially dissociate in water and include substances such as ammonia (NH3) and methylamine (CH3NH2).
Arrhenius Base Dissociation
The dissociation of an Arrhenius base in water is similar to the dissociation of an Arrhenius acid. When an Arrhenius base is added to water, it dissociates into its constituent ions, releasing hydroxide ions (OH-) into the solution. This process can be represented by the following equation: BOH → B+ + OH-, where BOH is the base and B+ is the conjugate acid. The extent of dissociation depends on the strength of the base, with strong bases dissociating completely and weak bases dissociating only partially.
| Base | Dissociation Constant (Kb) |
|---|---|
| Sodium hydroxide (NaOH) | 1.0 x 10^14 |
| Potassium hydroxide (KOH) | 1.0 x 10^14 |
| Calcium hydroxide (Ca(OH)2) | 5.0 x 10^13 |
| Ammonia (NH3) | 1.8 x 10^-5 |
What is the main difference between an Arrhenius acid and an Arrhenius base?
+The main difference between an Arrhenius acid and an Arrhenius base is the type of ion they produce in solution. Arrhenius acids produce hydrogen ions (H+), while Arrhenius bases produce hydroxide ions (OH-).
What is the dissociation constant (Ka) and how is it related to acid strength?
+The dissociation constant (Ka) is a measure of the strength of an acid, with higher values indicating stronger acids. The Ka value represents the equilibrium constant for the dissociation reaction of the acid in water.
Can you provide examples of strong and weak Arrhenius acids and bases?
+Strong Arrhenius acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3). Weak Arrhenius acids include acetic acid (CH3COOH) and carbonic acid (H2CO3). Strong Arrhenius bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2). Weak Arrhenius bases include ammonia (NH3) and methylamine (CH3NH2).
Meta Description: Learn about the Arrhenius acid definition, properties, and dissociation, as well as the Arrhenius base definition and dissociation. Understand the differences between strong and weak acids and bases, and how they relate to acid-base chemistry.